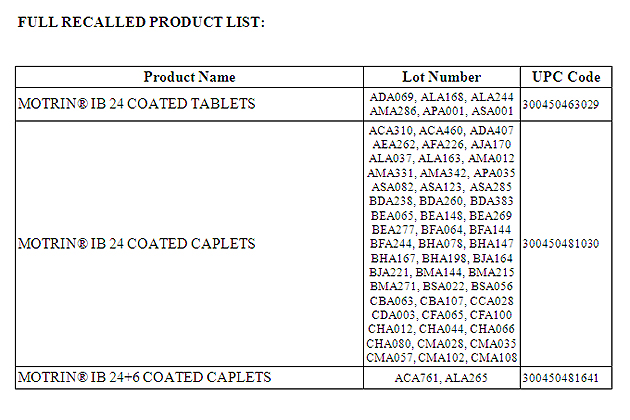
McNeil Consumer Healthcare Division of McNEIL-PPC, Inc. (McNeil) is voluntarily recalling certain lots of MOTRIN® IB 24 count COATED CAPLETS, MOTRIN® IB 24 count COATED TABLETS and MOTRIN® IB 24+6 count COATED CAPLETS from retailers. The products were distributed in the United States, Puerto Rico, Bahamas, Fiji, Belize, St. Lucia and Jamaica.
This is not a consumer level recall, which means that consumers do not need to dispose of or return the product. There is no safety concern if consumers continue taking the product in accordance with its label; however, it is possible there may be a delay in experiencing relief. This action is not being undertaken on the basis of adverse events.
McNeil is recalling these products because testing of product samples showed that some caplets may not dissolve as quickly as intended when nearing their expiration date. Out of an abundance of caution, we are recalling all the listed products since there is a chance they could experience a similar problem as they approach expiration. If you have any questions or concerns, please call our Consumer Call Center at 1-888-222-6036 (available Monday-Friday 8 a.m. to 8 p.m. Eastern Time).
The lot numbers (a full list is attached below) for the recalled product can be found on the side of the carton label.